T2 Biosystems Announces Exclusive U.S. Agreement with Cardinal Health to Sell its FDA-Cleared Direct-From-Blood Diagnostics for Rapid Detection of Sepsis-Causing Pathogens
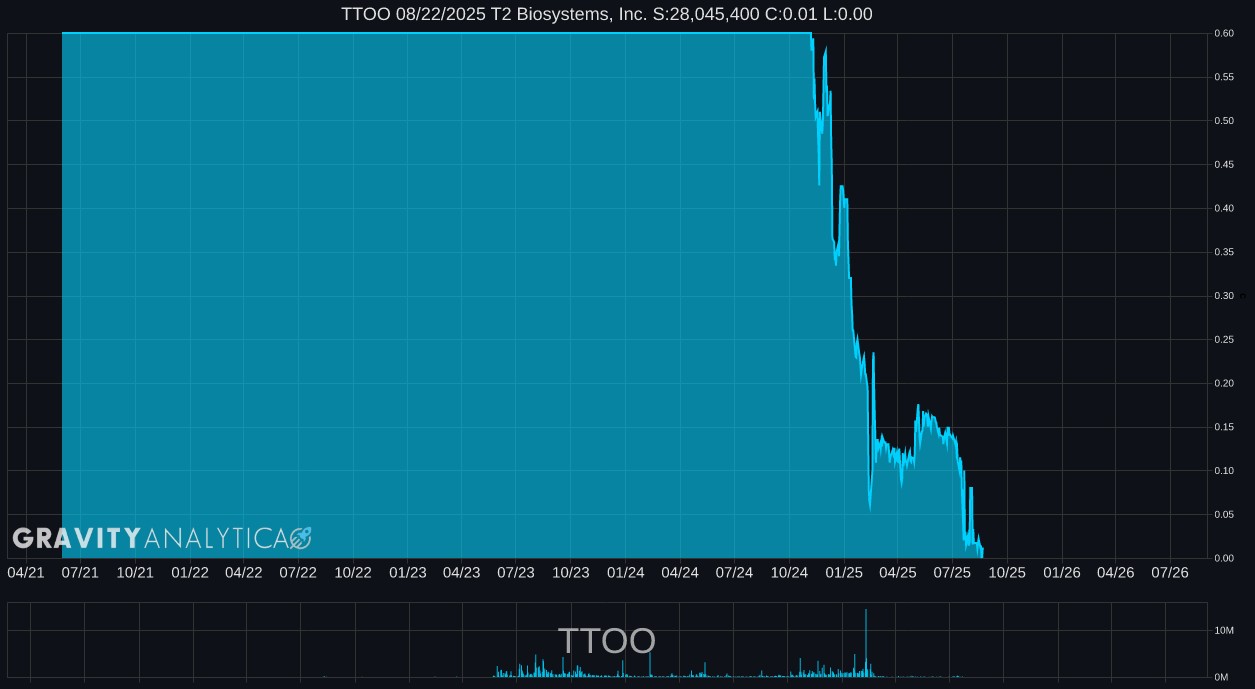
TTOO 10.07.2024
About Gravity Analytica
“We are thrilled to have entered into a distribution agreement with
Despite the widespread use of blood culture-based diagnostics to detect sepsis-causing pathogens and antibiotic susceptibility, sepsis remains the leading cause of death in
A
About
About
Centers for Disease Control https://www.cdc.gov/mmwr/volumes/72/wr/mm7234a2.htm- Buchman, T. G., Simpson, S. Q., Sciarretta, K. L., Finne, K. P., Sowers, N., Collier, M., ... & Kelman, J. A. (2020). Sepsis among medicare beneficiaries: 3. The methods, models, and forecasts of sepsis, 2012–2018.Critical care medicine,48(3), 302-318.https://journals.lww.com/ccmjournal/FullText/2020/03000/Sepsis_Among_Medicare_Beneficiaries__3__The.4.as
- Fingar K, Washington R. HCUP Statistical Brief #196.
November 2015 .Agency for Healthcare Research and Quality ,Rockville, MD .https://www.hcup-us.ahrq.gov/reports/statbriefs/sb196-Readmissions-Trends-High-Volume-Conditions.jsp - Gadre SK, et al. Chest. 2019;155(3):483-490.https://journal.chestnet.org/article/S0012-3692(18)32895-2/abstract
- Prescott H and Angus D. JAMA. 2018;319(1):91.https://jamanetwork.com/journals/jama/fullarticle/2667724
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements the likelihood that this collaboration will greatly expand our access to the
Investor Contact:

Source: T2 Biosystems, Inc.
