Single TearCare® Treatment Improved Functional Visual Outcomes as well as Signs and Symptoms of Patients with Dry Eye Disease in Investigator-Initiated Trial
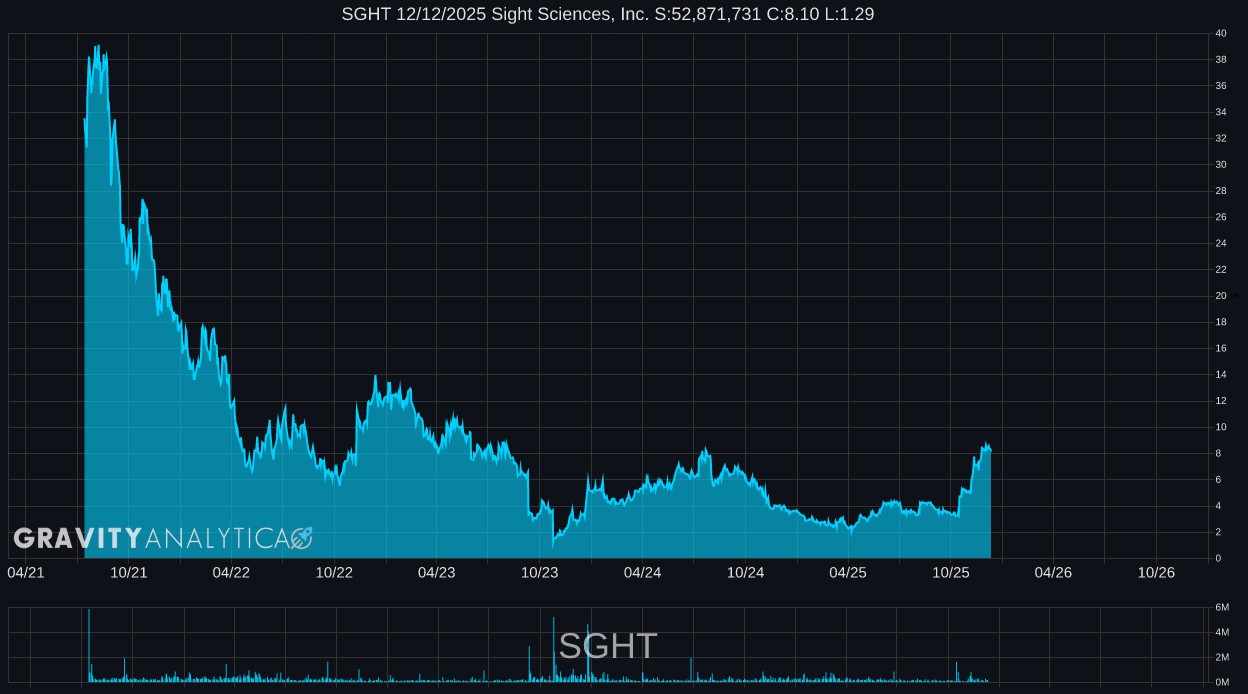
SGHT 10.14.2024
About Gravity Analytica
Recent News
- 01.14.2025 - Sight Sciences Announces Preliminary, Unaudited Fourth Quarter and Full Year 2024 Financial Highlights
- 01.07.2025 - Sight Sciences Announces Real-World 36-Month Study Confirming Long-Term Effectiveness of Standalone OMNI Surgical System in Managing Primary Open-Angle Glaucoma
Recent Filings
The study found improvements in reading speed, vision-related quality of life, and suggested potential benefits of an interventional approach for patients with dry eye disease
MENLO PARK, Calif., Oct. 14, 2024 (GLOBE NEWSWIRE) -- Sight Sciences, Inc. (Nasdaq: SGHT) (“Sight Sciences,” or the “Company”), an eyecare technology company focused on developing and commercializing innovative, interventional technologies that elevate the standard of care, announced today that a new study titled,Impact of TearCare on Reading Speed in Patients with Dry Eye Disease,has been published in the peer-reviewed journal Clinical Ophthalmology.
Key study findings reported in the study manuscript include:
- 52% of participants (N = 32) had a clinically significant improvement in reading speed after therapy with TearCare, defined as a greater than 10 words per minute increase in their International Reading Speed Texts (“IReST”) score. Improvements on the IReST and the Minnesota Low Vision Reading Test (“MNREAD”) reached statistical significance (p=0.012 and p=0.028, respectively).
- Ocular Surface Disease Index (“OSDI”) scores and National Eye Institute Visual Function Questionnaire 25 (“NEI-VFQ-25”) scores which measure symptoms and vision-related quality of life were both significantly improved after TearCare treatment (both p<0.001).
- Significant improvements in all dry eye disease (“DED”) sign metrics (p<.0.001), including tear break-up time, meibomian gland secretion score, and corneal fluorescein staining.
“A large portion of the patients I see in my practice experience significant issues from their dry eye disease. The disease can impact their quality of life and when it goes untreated patients can have reduced visual function,” said Preeya K. Gupta, MD, senior author and Director of Triangle Eye Consultants. “We are very pleased that this study demonstrated how a single in-office intervention with TearCare addressed the visual dysfunction associated with dry eye disease.”
This prospective single-center study evaluated the impact of TearCare treatment on clinical, vision-related quality of life, and functional visual outcome metrics in patients with meibomian gland disease (“MGD”) associated DED. The findings suggest patients who underwent a TearCare treatment exhibited improvements in vision-related quality of life and improved reading speeds after a single treatment. The study suggests that this interventional dry eye treatment should be considered and utilized to reduce the disease burden of MGD associated DED.
Authors and affiliations:Yilin Feng MD1, Nandini Venkateswaran MD1, Amanda Steele OD2, Eric D Rosenberg DO, MScEng3, Preeya K. Gupta MD2,4
- Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, USA
- Triangle Eye Consultants, Raleigh, NC, USA
- Department of Ophthalmology, New York Medical College, Valhalla, NY, USA
- Department of Ophthalmology, Tulane University, New Orleans, LA, USA
Paper Reference:Feng Y, Venkateswaran N, Steele A, Rosenberg ED, Gupta PK. Impact of TearCare on Reading Speed in Patients with Dry Eye Disease. Clin Ophthalmol. 2024;18:2873-2878
About Sight Sciences
Sight Sciences is an eyecare technology company focused on developing and commercializing innovative and interventional solutions intended to transform care and improve patients’ lives. Using minimally invasive or non-invasive approaches to target the underlying causes of the world’s most prevalent eye diseases, Sight Sciences seeks to create more effective treatment paradigms that enhance patient care and supplant conventional outdated approaches. Glaucoma is the world’s leading cause of irreversible blindness, and the Company’sOMNI®Surgical Systemis an implant-free glaucoma surgery technology (i) indicated in the United States to reduce intraocular pressure in adult patients with primary open-angle glaucoma; and (ii) CE-marked for the catheterization and transluminal viscodilation of Schlemm’s canal and cutting of the trabecular meshwork to reduce intraocular pressure in adult patients with open-angle glaucoma. TheSION®Surgical Instrumentis a bladeless, manually operated device used in ophthalmic surgical procedures to excise trabecular meshwork. The Company’sTearCare®Systemis 510(k) cleared in the United States for the application of localized heat therapy in adult patients with evaporative dry eye disease due to meibomian gland dysfunction (“MGD”), when used in conjunction with manual expression of the meibomian glands, enabling clearance of gland obstructions by physicians to address the leading cause of dry eye disease. Visit www.sightsciences.com for more information.
Sight Sciences, the Sight Sciences logo, and TearCare are trademarks of Sight Sciences registered in the United States. OMNI and SION are trademarks of Sight Sciences registered in the United States, European Union and other territories.
© 2024 Sight Sciences. All rights reserved.
Media contact:pr@SightSciences.com
Investor contact:Philip TaylorGilmartin Group415.937.5406Investor.Relations@Sightsciences.com
