Lumos Pharma Announces Two Abstracts Accepted for Oral Presentation at the 62ⁿᵈ Annual ESPE Meeting 2024
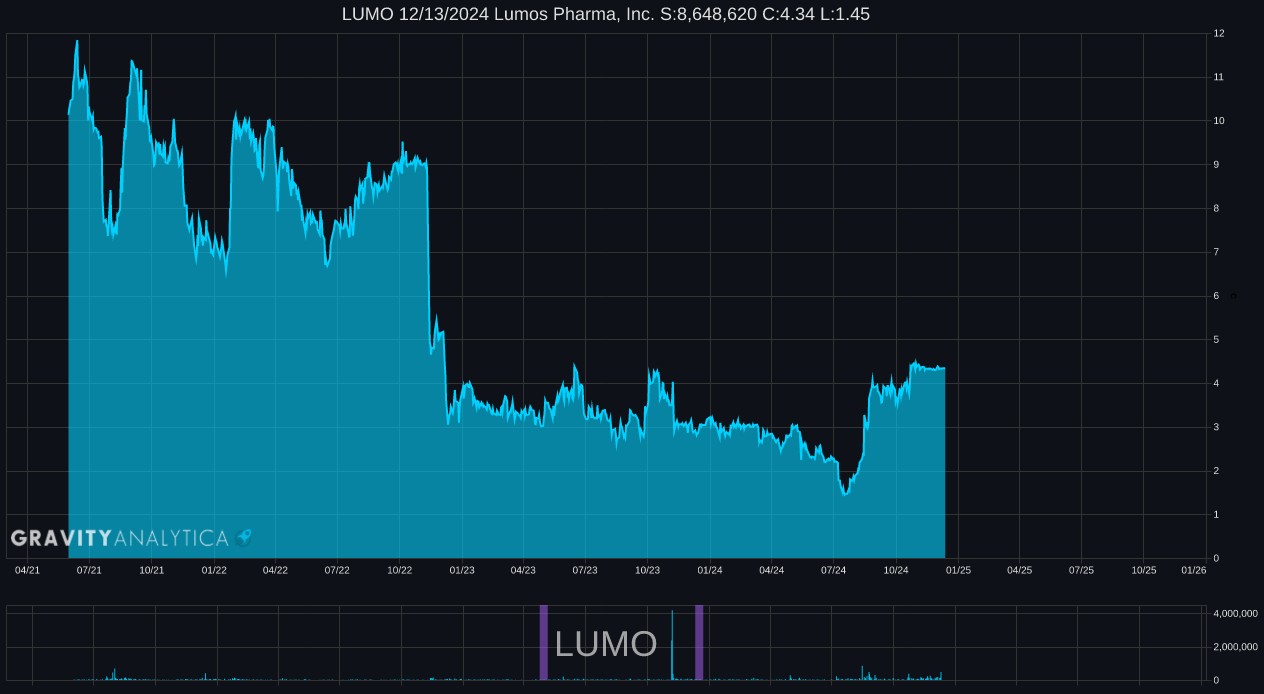
LUMO 10.30.2024
About Gravity Analytica
Recent News
Recent Filings
Two abstracts on data from the OraGrowtH Trials evaluating oral LUM-201 for Pediatric Growth Hormone Deficiency (PGHD) will be presented on
LUM-201 Oral Abstract Presentations:
- FC7.6 Abstract entitled,Growth, IGF-1 and IGFBP-3 Responses to Oral LUM-201 in OraGrowtH210 and OraGrowtH212 Trials in Pediatric Growth Hormone Deficiency (PGHD) Over 12 to 24 Months on Treatment(Elżbieta Petriczko, MD, PhD,et al)
- FC15.2 Abstract entitled,Amount and Pattern of Pulsatile GH Secretion Induced by the Oral Growth Hormone Secretagogue LUM-201 Is Related to Growth and IGF-1 Responses in Moderate Pediatric Growth Hormone Deficiency (PGHD)(
Peter E. Clayton , MD,et al)
About
Investor & Media Contact:
Source:

Source: Lumos Pharma, Inc.
