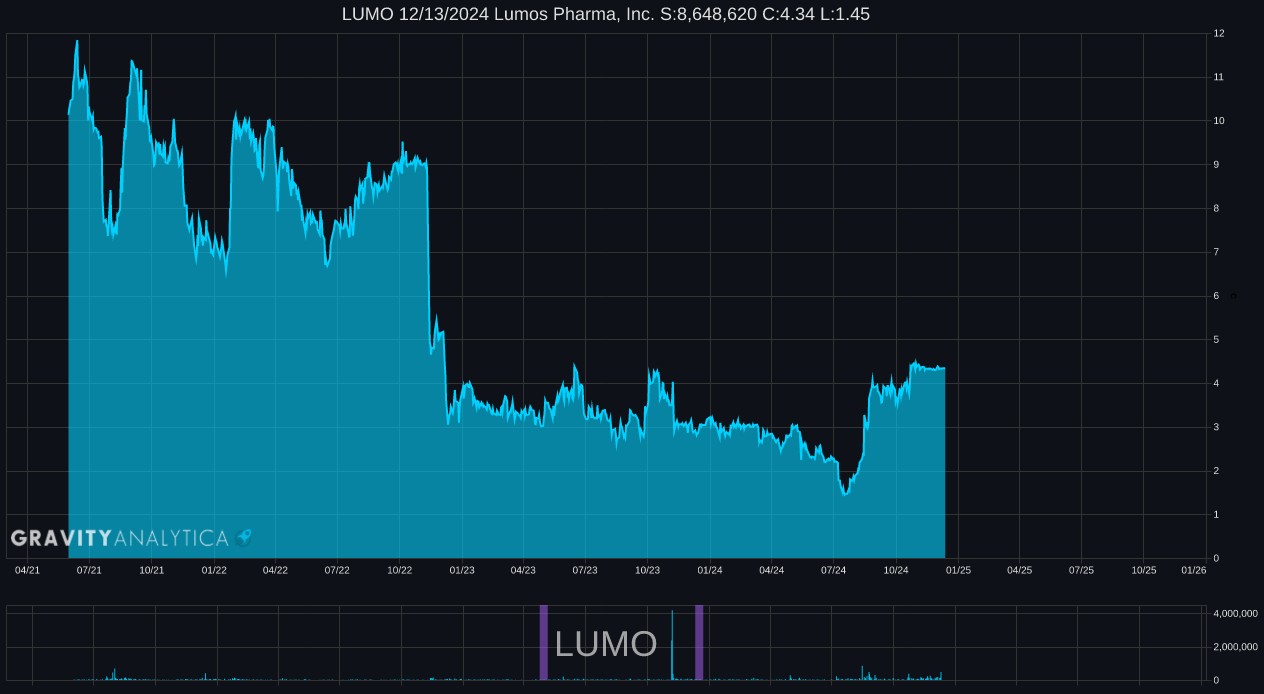
Lumos Pharma Enters into Definitive Merger Agreement with Double Point Ventures to Go Private via a Tender Offer of $4.25 Cash per Share Plus Contingent Value Rights (CVR)
LUMO 10.23.2024
Drug:LUM-201 LUM-201
Diseases:PGHD
About Gravity Analytica
Recent News
Recent Filings
Following a thorough review of financing and strategic alternatives, Lumos Pharma’s Board of Directors (the “Board”), with the assistance of the Board’s legal and financial advisors, unanimously determined that the acquisition by DPV is in the best interests of all
In addition,
Transaction Details
The Merger Agreement is structured as a tender offer by a wholly owned subsidiary of DPV for 100% of the outstanding shares of common stock of
The transactions contemplated by the Merger Agreement are not subject to any financing condition and DPV will fund the transactions from its existing cash resources.
Upon completion of the Merger,
In light of the Offer,
Advisors
Piper Sandler is serving as exclusive financial advisor to Lumos Pharma, and each of
Unaudited Financial Results for Q3 2024, Ending
Operating expenses for the third quarter ended
Cash balance as of
About
Lumos Pharma is a clinical stage biopharmaceutical company focused on the development and commercialization of therapeutics for rare diseases. The Company was founded and is led by a management team with longstanding experience in rare disease drug development. Lumos Pharma’s lead therapeutic candidate, LUM-201, is a novel, oral growth hormone (GH) secretagogue, seeking to transform the ~$4.7B global GH market from injectable to oral therapy. LUM-201 is currently being evaluated in multiple Phase 2 clinical studies in Pediatric Growth Hormone Deficiency (PGHD) and has received Orphan Drug Designation in both the US and EU. For more information, please visithttps://lumos-pharma.com/.
Cautionary Statement Regarding Forward Looking Statements
This release contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Lumos Pharma's beliefs and expectations and statements about the proposed Offer, merger and related transactions contemplated by the Merger Agreement (the "Transactions"), including the timing of and closing conditions to the Transactions; the potential effects of the proposed Transactions on
These forward looking statements may be identified by their use of forward-looking terminology including, but not limited to, “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” and “would,” and similar words expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance and involve risks and uncertainties that could cause actual results to differ materially from those projected, expressed or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: the possibility that the various closing conditions in the Merger Agreement may not be satisfied or waived, including uncertainties as to the percentage of shares of
The forward-looking statements contained in this release are made as of the date hereof, and
Additional Information and Where to Find It
The Offer described in this release has not yet commenced, and this release is for information purposes only and is neither a recommendation, nor an offer to purchase nor a solicitation of an offer to sell any shares of the common stock of Lumos Pharma or any other securities. On the commencement date of the Offer, a tender offer statement on Schedule TO, including an offer to purchase, a letter of transmittal and related documents, will be filed with the
Investor & Media Contact:
Source:

Source: Lumos Pharma, Inc.
