Pasithea Therapeutics Announces Opening of European Clinical Trial Sites and Completes Initial Dosing of Cohort 4
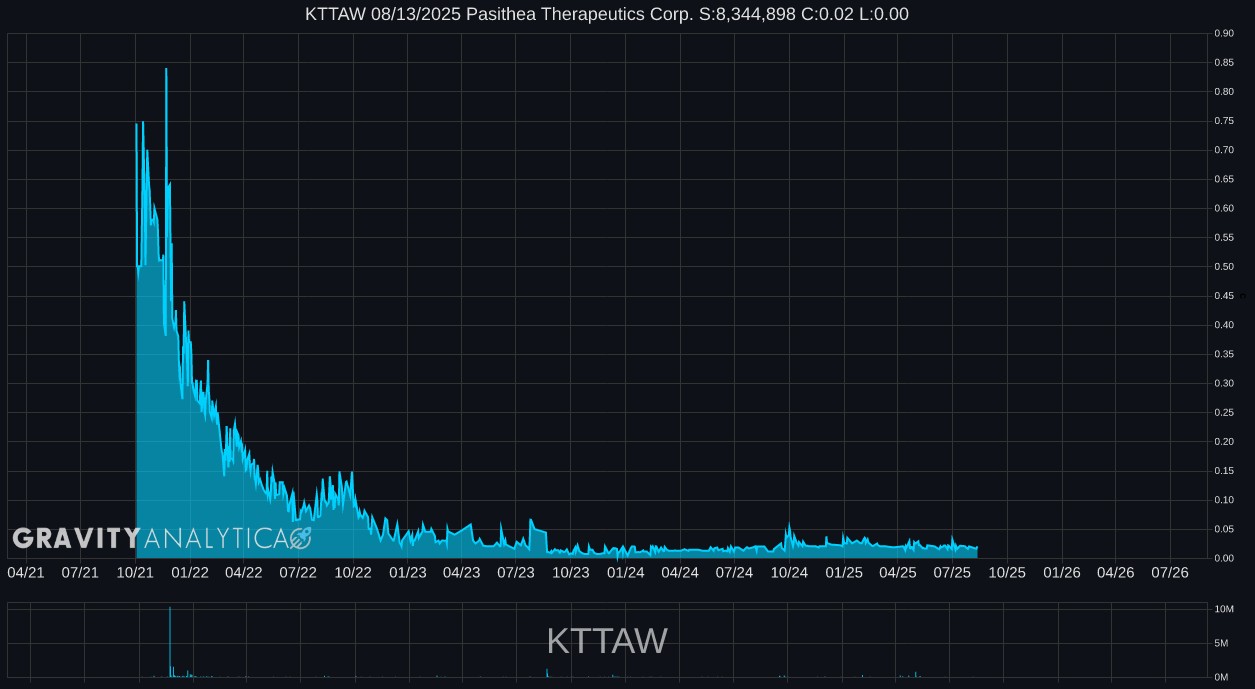
KTTAW 01.14.2025
About Gravity Analytica
Recent News
- 01.14.2025 - Pasithea Therapeutics Announces Opening of European Clinical Trial Sites and Completes Initial Dosing of Cohort 4
- 11.20.2024 - Pasithea Therapeutics Announces Positive Safety Review Committee (SRC) Recommendation from its ongoing Phase 1 Clinical Trial of PAS-004 in Advanced Cancer
- 09.26.2024 - Pasithea Therapeutics Announces $5 Million Private Placement Priced At-The-Market Under Nasdaq Rules
Recent Filings
Please be aware that the following content has been generated by an AI system and may contain errors, inconsistencies, or outdated information. It is provided as-is without any warranties or guarantees of accuracy. We strongly recommend using this content as a starting point for further research and consultation with relevant experts or authorities. We disclaim any liability for damages or losses resulting from the use or reliance on this content.Please note that this is a beta version of the Gravity Analytica LLC’s AI Service which isstill undergoing final testing before its official release. Theplatform, its software and all content found on it are provided on an“as is” and “as available” basis. Gravity Analytica LLC does not give any warranties,whether express or implied, as to the suitability or usability of thisservice, webpage, or its software or any of its content.Should you encounter any bugs, glitches, lack of functionality orother problems on the website, please let us know immediately so wecan rectify these accordingly. Your help in this regard is greatlyappreciated! You can write to us at this addressteam@gravityanalytica.com