Immatics Announces Multiple Presentations at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC) on TCR-T Therapy Candidates Targeting PRAME
IMTXW 11.08.2024
Two oral presentations and multiple posters on clinical andpreclinical-stage candidates to be presented at SITC, demonstratingthe strength of Immatics’TCR-TPRAME franchise to target solid cancers
- ACTengine® IMA203 demonstrates 54% cORR, 12.1 months mDOR and 6 months mPFS in heavily pretreated metastatic melanoma patients and >1-year mPFS in patients with deep responses; Company plans to start its randomized-controlled Phase 3 SUPRAME trial in
December 2024 to evaluate IMA203 in second-line or later metastatic melanoma - Next-generation ACTengine® IMA203CD8 TCR-T cell therapy targeting PRAME demonstrates enhanced pharmacology and potency per cell; Phase 1a dose escalation reinitiated to target higher doses, positioning this TCR-T candidate for future development in solid cancers with medium-level PRAME copy numbers, such as ovarian cancer, endometrial cancers and triple-negative breast cancer
- A first update on Immatics’ Bispecific TCER® IMA402 targeting PRAME and initial clinical data from the ongoing Phase 1a dose escalation trial is expected to be reported by year-end
All dates and times of Immatics’ upcoming oral and poster presentations at the 39thAnnual Meeting of the
“Immatics remains fully focused on the clinical development of our most advanced lead product candidate, IMA203, in second-line or later metastatic melanoma patients. We look forward to the initiation of SUPRAME, the registration-enabling Phase 3 trial, in December,” said Dr.
ACTengine® IMA203 Monotherapy Phase 1b Trial - Clinical Data and Development Path Summary
On
The data announced today include all infused patients in the Phase 1b dose expansion part of the trial (N=412), consisting of the 28 melanoma patients reported on
IMA203 monotherapy has maintained a favorable tolerability profile with no treatment-related Grade 5 events in the entire safety population (N=703Phase 1a and Phase 1b patients across all dose levels and all tumor types).
Best Overall Response for IMA203 in Dose Expansion in All Indications (N=41#)
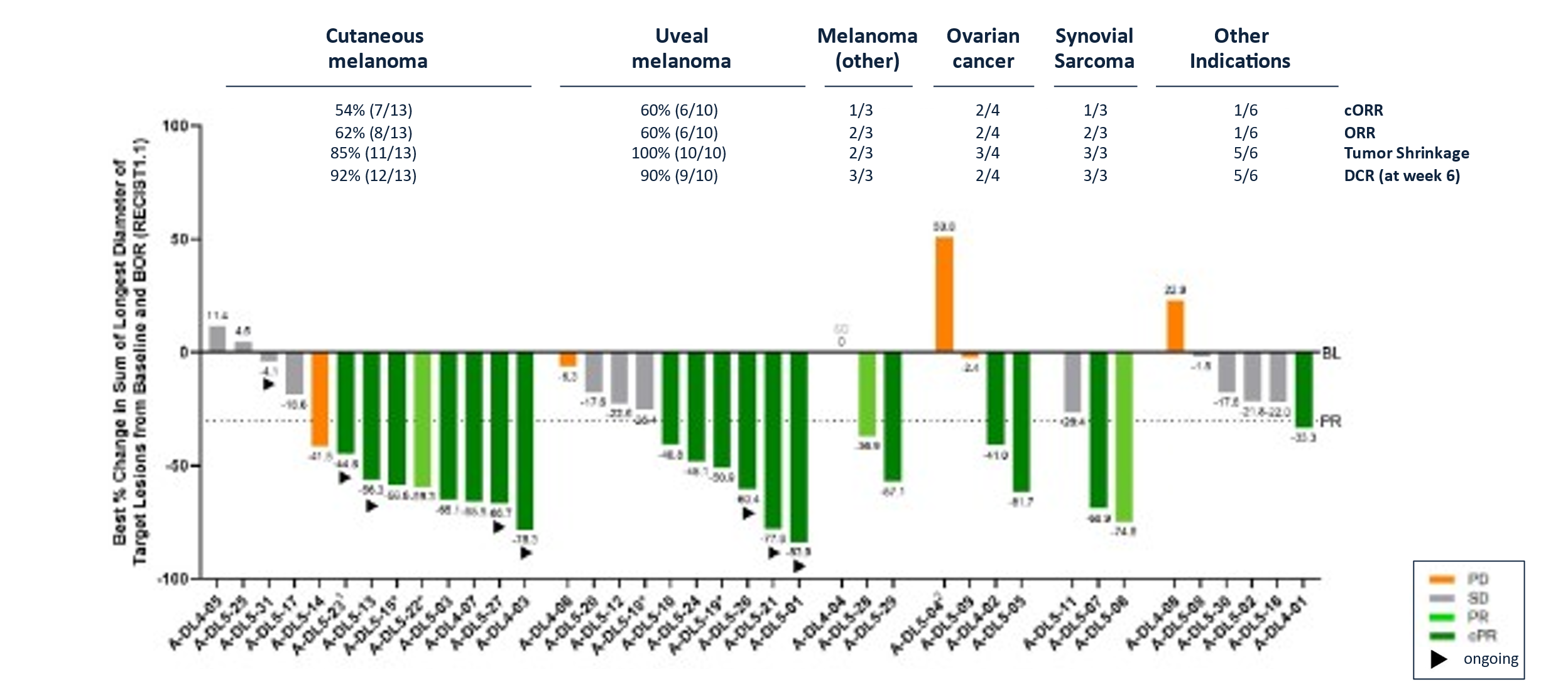
Data cut-off
Development Path for IMA203Based on the Phase 1b data, the Company is on track to commence SUPRAME, the registration-enabling Phase 3 randomized-controlled clinical trial in melanoma for IMA203, in
SUPRAME will evaluate IMA203 targeting PRAME in 360 HLA-A*02:01-positive patients with second-line or later (2L+) unresectable or metastatic melanoma who have received prior treatment with a checkpoint inhibitor. Patients will be randomized 1:1 for IMA203 or investigator’s choice of selected approved treatments in the 2L+ setting, including nivolumab/relatlimab, nivolumab, ipilimumab, pembrolizumab, lifileucel (US only) or chemotherapy. The primary endpoint for full approval will be median PFS and secondary endpoints will include objective response rate, safety, duration of response, no overall survival detriment and patient-reported outcomes.
Patient enrollment for SUPRAME is forecast to be completed in 2026, and a pre-specified interim analysis is planned for early 2026.
ACTengine® IMA203CD8 (GEN2) Monotherapy Phase 1 Dose Escalation Trial - Patient Population & Clinical Data Summary
Patient population:Heavily pretreated patients with solid tumorsAs of data cut-off on
Safety:Treatment with IMA203CD8 demonstrates a manageable tolerability profile across dose levelsIMA203CD8 monotherapy has maintained a manageable tolerability profile in the 44 patients treated. The most frequent adverse events at or above Grade 3 were expected cytopenia associated with lymphodepletion. Some patients also experienced mild to moderate CRS (Grade 1: 36% Grade 2: 48% Grade 3: 11% Grade 4: 2%).
As previously reported, two patients experienced dose-limiting toxicities at dose level 4b, which prompted a dosing adjustment to dose level 4a. After further assessing the tolerability profile of IMA203CD8 in additional patients treated at dose level 4a, the eligibility criteria and the IL-2 dose regimen were modified, and dose escalation beyond dose level 4a was reinitiated. One Grade 5 adverse event classified as possibly related to treatment with IMA203CD8 was also observed as reported previously in
Anti-tumor activity and durability:Deep and durable objective responses observed
- As of data cut-off on
September 30, 2024 , 10 of 17 responses were ongoing, of which three confirmed responses were ongoing at 14+, 15+ and 24+ months. - Of note, these patients had been treated at substantially lower doses compared to IMA203 (GEN1), i.e. in a range of 0.2-0.48x109TCR-T cells/m2BSA (dose level 3) to 0.801-1.2x109TCR-T cells/m2BSA (dose level 4c) T cells infused.
- Deep responses with ≥50% tumor size reduction were observed in 11 out of 17 responders. This group included two patients with complete response of target lesions, of which one patient showed a complete metabolic response according to PET-CT scan6.
- 41% (14/34) confirmed objective response rate (cORR) and 41% (17/41) objective response rate (ORR).
- Median duration of response (mDOR) of 9.2 months at a median follow-up (mFU) of 13.1 months.
- Tumor shrinkage7of 84% (32/38) and disease control rate8at week 6 of 85% (34/40).
Translational data:Opportunity of IMA203CD8 in medium-level PRAME expressing indicationsTranslational data indicate that PRAME expression level is associated with clinical activity in IMA203 and IMA203CD8 treated patients. Both IMA203 and IMA203CD8 achieved deep responses despite IMA203CD8 patients receiving lower product doses. Based on the enhanced pharmacology of IMA203CD8, the evaluation of higher doses of IMA203CD8 in the ongoing dose escalation trial opens the possibility of addressing hard-to-treat solid tumor indications with a medium-level of PRAME copy numbers, such as ovarian cancer, endometrial cancers and triple-negative breast cancer.
Preclinical Data on New Approaches for TCR-T Based Cell Therapies
As part of Immatics’ long-term strategy to expand its PRAME franchise, the Company has conducted preclinical studies for the potential future clinical development of next-generation TCR-T-based cell therapies targeting PRAME to further enhance the efficacy and durability of IMA203. These efforts include the evaluation of TCR-T cells armored with membrane-bound IL-15 (mbIL15) targeting tumor types with low PRAME copy numbers, such as squamous non-small-cell lung cancer and squamous head and neck cancers. In addition, the Company is developing an allogeneic cell therapy approach to further increase commercial attractiveness and to reach patients quickly with its next-generation off-the-shelf cell therapy, ACTallo®. The preclinical data will be presented during poster sessions at SITC.
About ACTengine® IMA203, IMA203CD8 and Target PRAMEACTengine®IMA203 is Immatics’ most advanced TCR-based autologous cell therapy that is directed against an HLA-A*02-presented (human leukocyte antigen) peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers. PRAME is homogeneously and specifically expressed in tumor tissue and Immatics’ PRAME peptide is present at a high copy number per tumor cell. The peptide has been identified and characterized by Immatics’ proprietary mass spectrometry-based target discovery platform, XPRESIDENT®. Through its proprietary TCR discovery and engineering platform
ACTengine®IMA203 TCR-T is currently being evaluated as a monotherapy in a Phase 1 clinical trial in patients with solid tumors expressing PRAME, such as cutaneous melanoma. An IMA203 registration-enabling randomized controlled Phase 3 trial, “SUPRAME,” is planned to commence in
ACTengine® IMA203 TCR-T is also currently being evaluated in Phase 1 IMA203CD8 (GEN2) monotherapy, where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor.
- END -
About
Forward-Looking StatementsCertain statements in this press release may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND or CTA filing for pre-clinical stage product candidates, the timing of BLA filings for clinical stage product candidates, estimated market opportunities of product candidates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by
For more information, please contact:
| Media | |
| Phone: +49 171 3512733 | |
| immatics@trophic.eu |
| Head of Strategy | |
| Phone: +1 346 319-3325 | |
| InvestorRelations@immatics.com |
1Includes one patient who started lymphodepletion but did not receive IMA203 TCR-T cells.2All infused patients, first tumor assessment post infusion pending for 2/28 melanoma patients at data-cut.3All patients who started lymphodepletion as of the data cut-off on
Attachment
 https://immatics.com/
https://immatics.com/