Avadel Announces Grant of Inducement Awards Under Nasdaq Listing Rule 5635(c)(4)
AVDL 01.06.2025
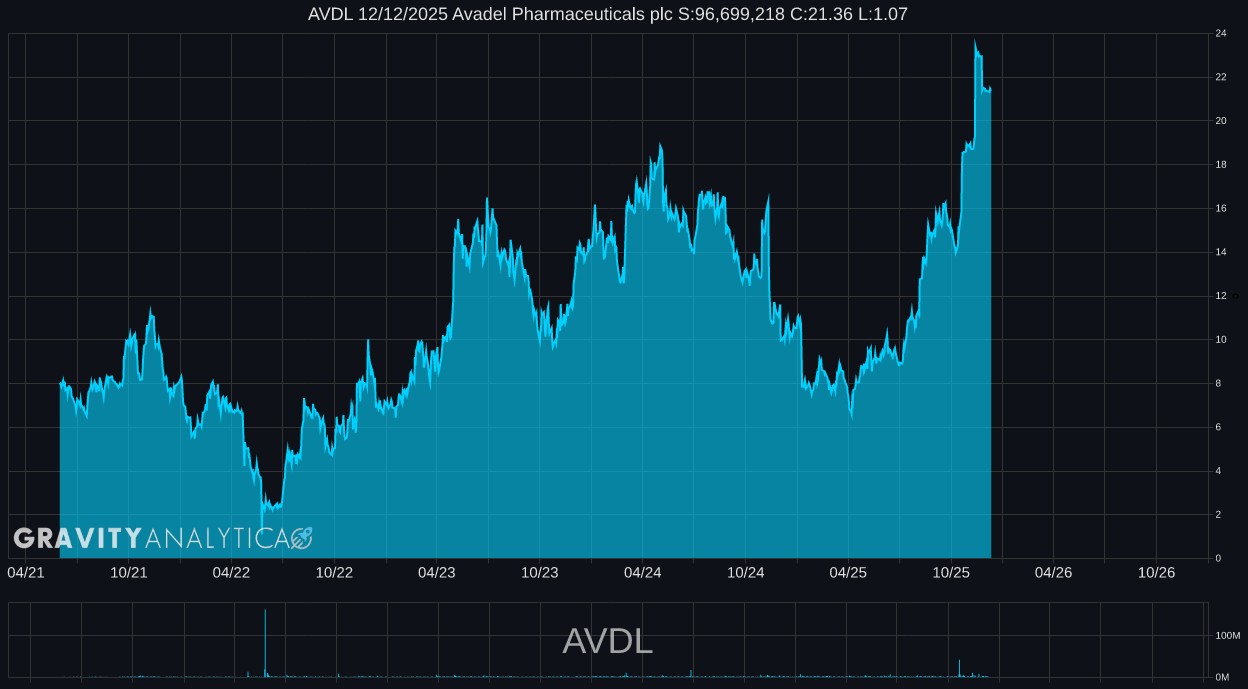
About Gravity Analytica
Recent News
- 01.22.2025 - Avadel Pharmaceuticals Appoints Sev Melkonian as Vice President of Patient Services, Distribution, and Reimbursement
- 01.08.2025 - Avadel Pharmaceuticals Business Update Call
- 01.08.2025 - Avadel Announces Preliminary 2024 Results and 2025 Commercial Priorities to Accelerate the LUMRYZ Launch
Recent Filings
About Avadel Pharmaceuticals plc
Investor Contact:
Media Contact:

Source: Avadel Pharmaceuticals plc