Astria Therapeutics to Present at Upcoming Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting
ATXS 10.30.2024
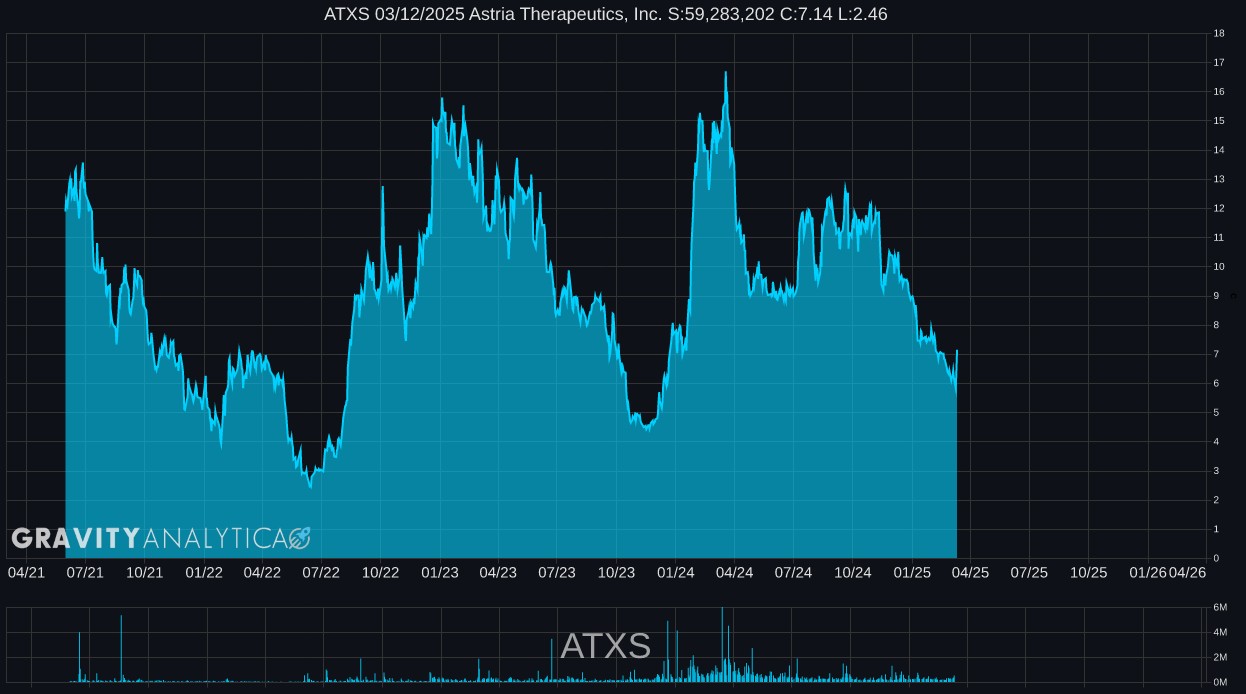
About Gravity Analytica
Recent News
- 01.23.2025 - Astria Therapeutics Announces Initiation of Phase 1a Trial of STAR-0310, a Potential Best-in-Class Monoclonal Antibody OX40 Antagonist for the Treatment of Atopic Dermatitis
- 01.13.2025 - Astria Therapeutics Announces Design of ALPHA-ORBIT Pivotal Phase 3 Trial of Navenibart in HAE
- 01.03.2025 - Astria Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
Recent Filings
About
View source version onbusinesswire.com:https://www.businesswire.com/news/home/20241030578125/en/
Source: