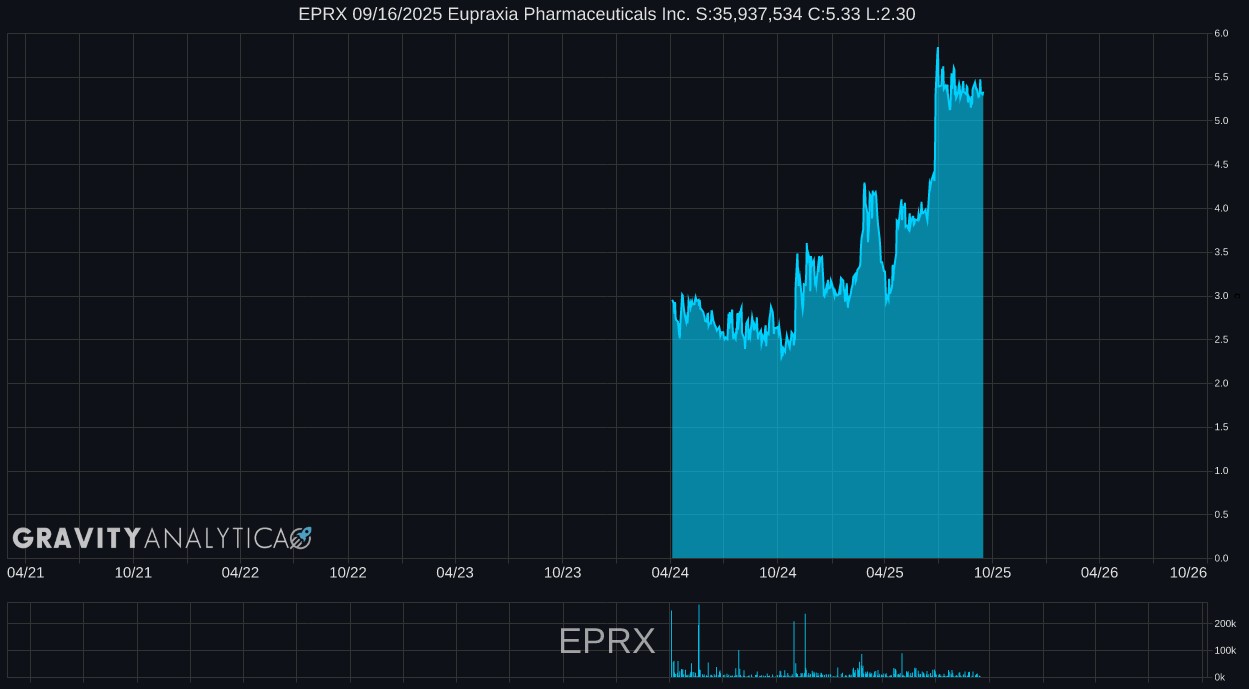
Eupraxia Pharmaceuticals Inc. - EPRX
About Gravity Analytica
Recent News
- 09.29.2025 - Eupraxia Pharmaceuticals Announces Positive Data from Highest-Dose Cohort in the Ongoing RESOLVE Trial in Eosinophilic Esophagitis, and Plans for Expansion of EP-104GI Development Programs
- 09.29.2025 - Eupraxia Pharmaceuticals Announces Positive Data from Highest-Dose Cohort in the Ongoing RESOLVE Trial in Eosinophilic Esophagitis, and Plans for Expansion of EP-104GI Development Programs
- 09.24.2025 - Eupraxia Pharmaceuticals Announces Closing of US$80.5 Million Public Offering Including Full Exercise of Underwriter Option
- 09.24.2025 - Eupraxia Pharmaceuticals Announces Closing of US$80.5 Million Public Offering Including Full Exercise of Underwriter Option
- 09.23.2025 - Eupraxia Pharmaceuticals Announces Pricing of US$70 Million Public Offering of Common Shares
- 09.23.2025 - Eupraxia Pharmaceuticals Announces Pricing of US$70 Million Public Offering of Common Shares
- 09.22.2025 - Eupraxia Pharmaceuticals Announces Proposed Public Offering of Common Shares
- 09.22.2025 - Eupraxia Pharmaceuticals Announces Proposed Public Offering of Common Shares
Recent Filings
- 09.29.2025 - 6-K Report of foreign issuer [Rules 13a-16 and 15d-16]
- 09.29.2025 - EX-99.1 EX-99.1
- 09.26.2025 - SCHEDULE 13D/A General Statement of Acquisition of Beneficial Ownership
- 09.24.2025 - 6-K Report of foreign issuer [Rules 13a-16 and 15d-16]
- 09.24.2025 - EX-99.1 EX-99.1
- 09.23.2025 - EX-99.1 EX-99.1
- 09.23.2025 - EX-99.1 EX-99.1
- 09.23.2025 - 6-K Report of foreign issuer [Rules 13a-16 and 15d-16]
- 09.23.2025 - SUPPL Voluntary Supplemental Material by Foreign Issuers [Section 11(a)]
- 09.23.2025 - 6-K Report of foreign issuer [Rules 13a-16 and 15d-16]